Choline Chloride Hydrogen Bond Acceptor . the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors.
from ww2.chemistry.gatech.edu
choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline.
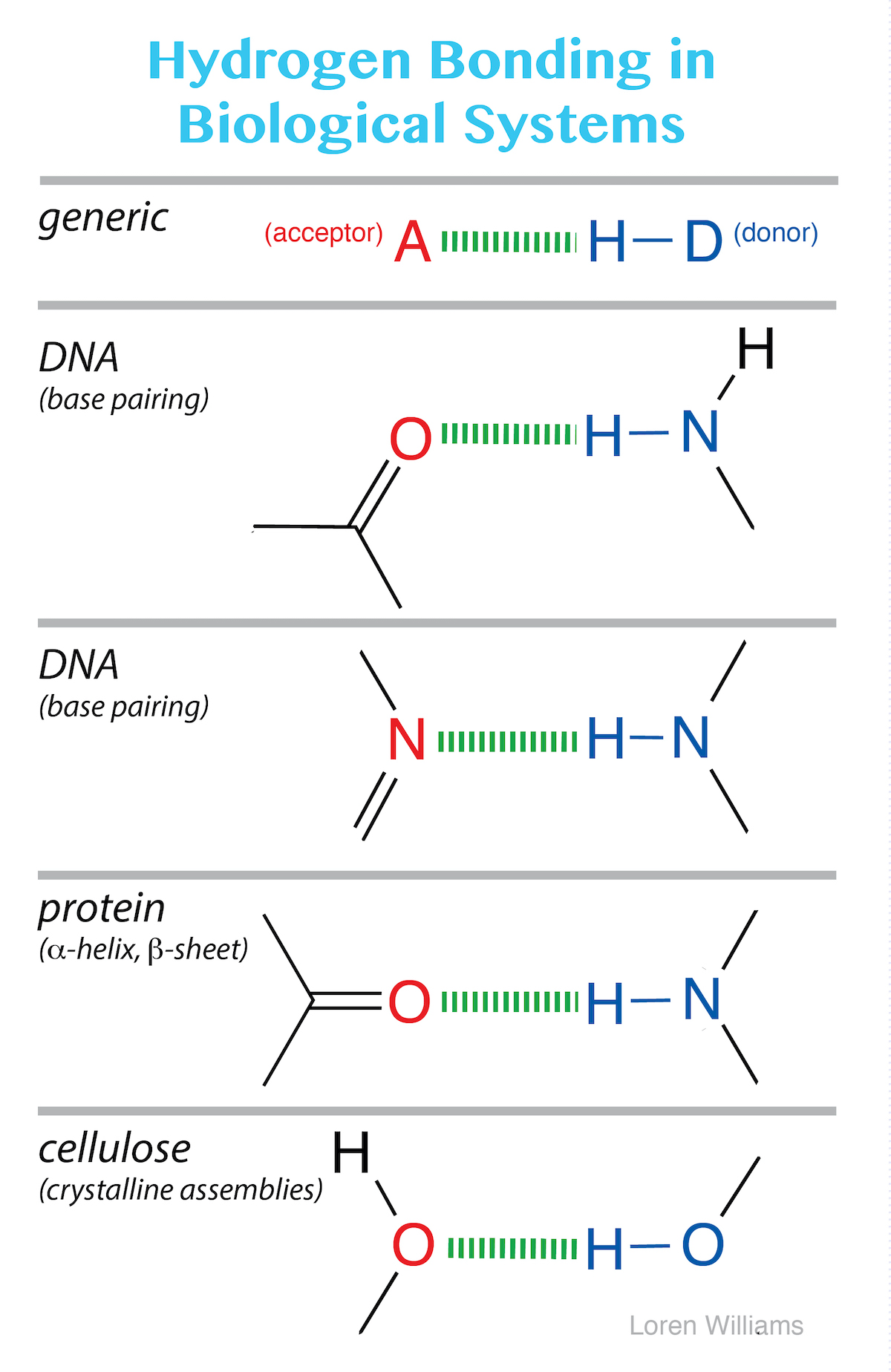
Molecular Interactions (Noncovalent Interactions)
Choline Chloride Hydrogen Bond Acceptor to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors.
From www.mdpi.com
Sustainability Free FullText Physicochemical Properties of Choline Choline Chloride Hydrogen Bond Acceptor in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. the. Choline Chloride Hydrogen Bond Acceptor.
From cartoondealer.com
Cholin Cartoons, Illustrations & Vector Stock Images 17 Pictures to Choline Chloride Hydrogen Bond Acceptor this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride. Choline Chloride Hydrogen Bond Acceptor.
From ww2.chemistry.gatech.edu
Molecular Interactions (Noncovalent Interactions) Choline Chloride Hydrogen Bond Acceptor choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl).. Choline Chloride Hydrogen Bond Acceptor.
From www.mdpi.com
IJMS Free FullText Deep Eutectic Solvent as Green Solvent in Choline Chloride Hydrogen Bond Acceptor to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. in this study, we. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Structure of the hydrogen bond donors (HBDs), and hydrogen bond Choline Chloride Hydrogen Bond Acceptor the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
a Choline chloride forming hydrogen bonds with starch, b Choline Choline Chloride Hydrogen Bond Acceptor choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). to prepare. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Chemical structures of the DESs investigated in this research, using Choline Chloride Hydrogen Bond Acceptor in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. in this. Choline Chloride Hydrogen Bond Acceptor.
From www.mdpi.com
Antioxidants Free FullText Integration of Choline ChlorideBased Choline Chloride Hydrogen Bond Acceptor this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the.. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Raman spectra of choline chloride and reline in the symmetric Choline Chloride Hydrogen Bond Acceptor in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. this work employed molecular dynamics techniques to investigate the structure and dynamics of a. Choline Chloride Hydrogen Bond Acceptor.
From www.orientjchem.org
Comparative Assessment of Choline ChlorideBased Deep Eutectic Solvents Choline Chloride Hydrogen Bond Acceptor this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Components of the tested DES mixtures Choline chloride (i) and betaine Choline Chloride Hydrogen Bond Acceptor in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. this. Choline Chloride Hydrogen Bond Acceptor.
From pubs.acs.org
Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Choline Chloride Hydrogen Bond Acceptor choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents (dess), and the. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of. Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Molecular structures of hydrogen bond acceptor (HBA) and hydrogen bond Choline Chloride Hydrogen Bond Acceptor the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor. Choline Chloride Hydrogen Bond Acceptor.
From pubs.acs.org
Hydrogen Bond Donor/Acceptor CosolventModified Choline ChlorideBased Choline Chloride Hydrogen Bond Acceptor the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating. Choline Chloride Hydrogen Bond Acceptor.
From www.nature.com
Insights into the interactions and dynamics of a DES formed by phenyl Choline Chloride Hydrogen Bond Acceptor the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline. Choline Chloride Hydrogen Bond Acceptor.
From www.mdpi.com
Molecules Free FullText Polyalcohols as HydrogenBonding Donors in Choline Chloride Hydrogen Bond Acceptor to prepare eutectic solvents, chcl, as hydrogen bond acceptor (hba), was mixed with different hydrogen bond donors. this work employed molecular dynamics techniques to investigate the structure and dynamics of a des system composed of choline. in this regard, the effect of different structures of the hydrogen bond donor (hbd) in choline chloride (chcl). the interaction. Choline Chloride Hydrogen Bond Acceptor.
From www.youtube.com
Tweaking Behavior of Hydrogen Bond Donor in Choline ChlorideBased Deep Choline Chloride Hydrogen Bond Acceptor in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. in this regard, the effect of different structures of the hydrogen bond donor (hbd). Choline Chloride Hydrogen Bond Acceptor.
From www.researchgate.net
Chitin dissolution by using choline chloride as the hydrogen bond Choline Chloride Hydrogen Bond Acceptor in this study, we reported a typical hydrogen bond acceptor of choline chloride (chcl) to tailor the hydrogen‐donating environment of the. the interaction of the chloride anion with the methyl protons (h4) and methylene protons (h3) of chcl as well as the strong. choline chloride (chcl) is a common hydrogen bond acceptor (hba) for deep eutectic solvents. Choline Chloride Hydrogen Bond Acceptor.